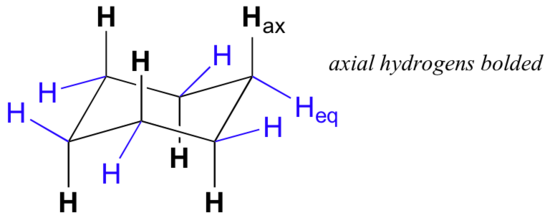
This is the currently selected item. for all the cyclic molecules we've dealt with so far, we've just drawn them as rings. for example, for cyclohexane we've literally just drawn it as a hexagon. so we've drawn cyclohexane like that. now, we know from the last several videos that all the bonds for. This organic chemistry video tutorial focuses on the chair conformation of cyclohexane. it shows how to draw the most stable conformation. it contains a few notes, examples, and practice problems. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a "chair". in this conformation there is no torsional strain at all, and as we shall see later, no strain of any kind. cyclohexane is unique in being the only cyclic hydrocarbon which is completely strain-free..
Cyclohexane’s chair, twist and boat conformations repeat the process with cyclohexane, but make sure the optimization limit is set to normal. the greater flexibility of this molecule will be evident by the fact that marvinsketch finds several conformations. the lowest energy form is the most important.. We don't have to worry about any torsional strain, so the chair conformation is the most stable conformation for cyclohexane. next we'll take a look at the boat conformation. here we have the boat conformation of cyclohexane, if you look at the carbons it looks a little bit like a boat.. Chair-chair interconversion like other conformations we have studied, chair conformations are in a state of constant flux. because all the c-c bonds are interconnected, they cannot rotate independently but have to move together. for example, one end of the chair could "flip up" to put the cyclohexane ring in a boat conformation..

Comments
Post a Comment