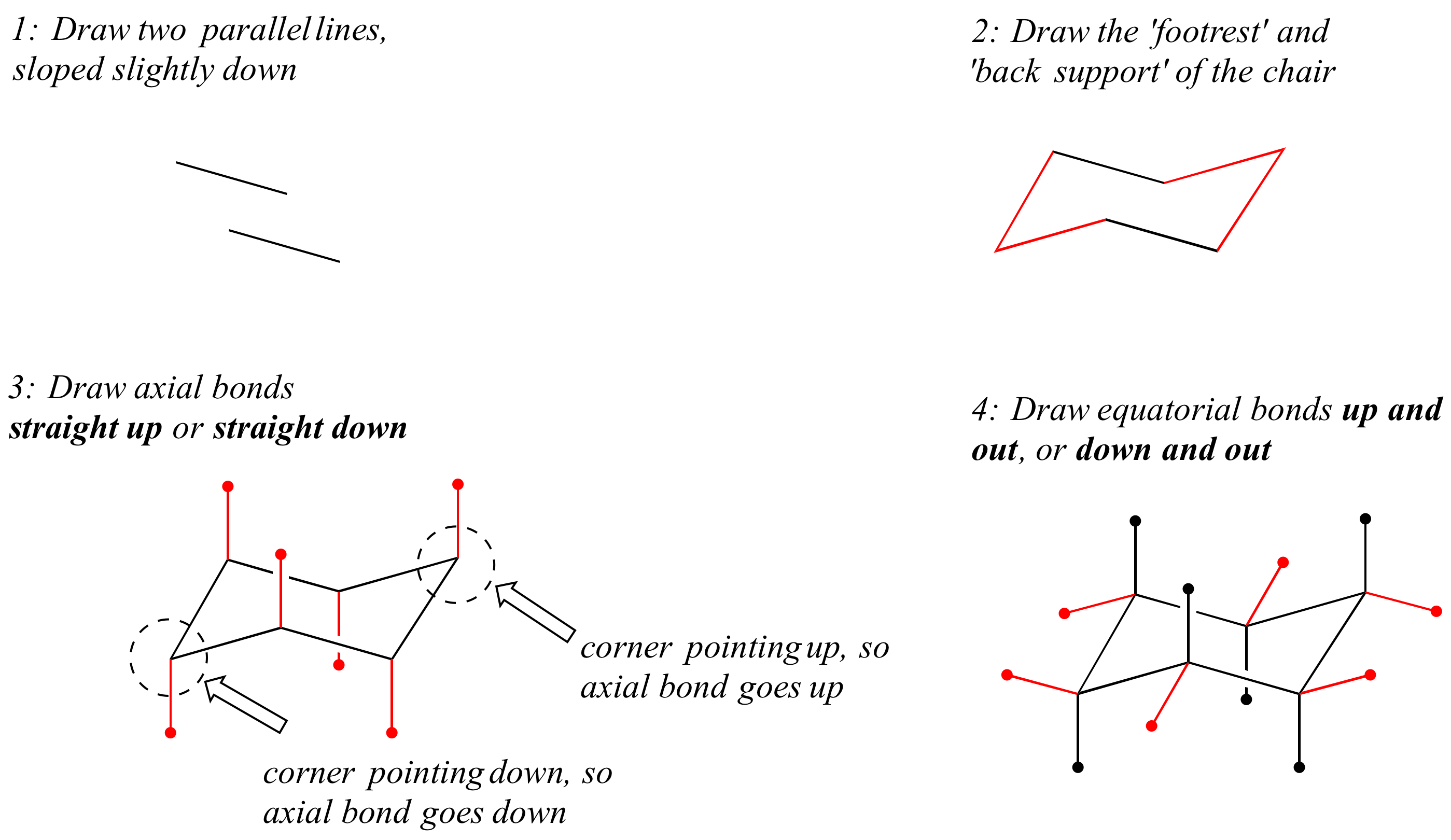
Chair and boat shapes for cyclohexane. chair and boat shapes for cyclohexane. science organic chemistry alkanes, cycloalkanes, and the reason why it's useful to know that name is when we talk about the different configurations, the different chair and boats, whether something is equatorial or axial can change if this were to flip up, or. This organic chemistry video tutorial focuses on the chair conformation of cyclohexane. it shows how to draw the most stable conformation. it contains a few notes, examples, and practice problems. The chair, boat, and twist-boat conformations show the angles much closer to the ideal 109.5 o, and these are the shapes that most cyclohexane molecules are actually found to be in. the three.
The twist-boat conformation is 5. and are impossible to isolate. compare this to the chair with all bonds staggered and complete absence of torsional strain and the twist-boat with 4 out 6 bonds partially eclipsed. the torsional strain in the boat conformation has a maximum value because two of the carbon bonds are eclipsed.. Science organic chemistry alkanes, cycloalkanes, and functional groups conformations of cycloalkanes. conformations of cycloalkanes. chair and boat shapes for cyclohexane. double newman diagram for methylcyclohexane. stability of cycloalkanes. conformations of cyclohexane. drawing chair conformations. conformations of cycloalkanes.. To gain more stability, cyclohexane adopts the chair conformation instead. the chair conformation is a six-membered ring in which atoms 2, 3, 5, and 6 lie in the same plane, atom 1 lies above the plane, and atom 4 lies below the plane. we will examine how to draw and number the structure later. with this conformation, the bond angles are 110.9.
Comments
Post a Comment