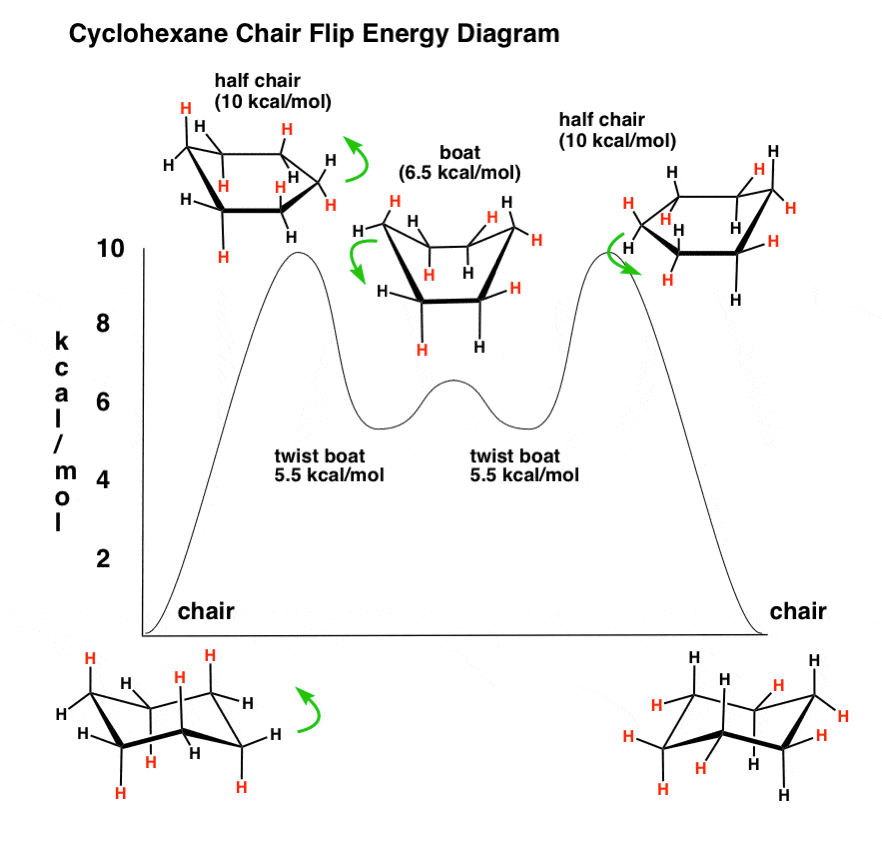
Chair and boat shapes for cyclohexane. chair and boat shapes for cyclohexane. stability of cycloalkanes. conformations of cyclohexane. the different chair and boats, whether something is equatorial or axial can change if this were to flip up, or vice versa, and things like that.. The ground state conformation of cyclohexane is a fully staggered conformation which is shaped somewhat like a "chair". in this conformation there is no torsional strain at all, and as we shall see later, no strain of any kind. cyclohexane is unique in being the only cyclic hydrocarbon which is completely strain-free.. In the video you'll see the model kit next to the the drawings of cyclohexane in chair conformation to help you understand stability. links mentioned in video links & resources mentioned in this.
Another conformation of cyclohexane exists, known as boat conformation, but it interconverts to the slightly more stable chair formation. if cyclohexane is mono-substituted with a large substituent, then the substituent will most likely be found attached in an equatorial position, as this is the slightly more stable conformation.. We don't have to worry about any torsional strain, so the chair conformation is the most stable conformation for cyclohexane. next we'll take a look at the boat conformation. here we have the boat conformation of cyclohexane, if you look at the carbons it looks a little bit like a boat.. Chair-chair interconversion like other conformations we have studied, chair conformations are in a state of constant flux. because all the c-c bonds are interconnected, they cannot rotate independently but have to move together. for example, one end of the chair could "flip up" to put the cyclohexane ring in a boat conformation..
Comments
Post a Comment